Eleveld TCI on Agilia SP TIVA syringe pump
It is the responsibility of the healthcare professional to understand and have been trained on the use of any TCI model used on the Agilia SP TIVA.
Please ensure you understand the clinical implications of use of the Eleveld Propofol and Remifentanil TCI models, including the difference in use with and without opioids for Propofol, before using the TCI model.
The pharmacokinetic models included in the Agilia SP TIVA pump were not developed specifically for a device, but they were established and validated by numerous clinical studies.
Below are the base Target Controlled Infusion (TCI) models included as an option with all Agilia SP TIVA pumps as part of your initial purchase:

a. B.Marsh, M.White, N. Morton, G.N.C. Kenny. Pharmacokinetic model driven infusion of propofol in children. British Journal of Anesthesia. 1991, 67, pp. 41-48.
b. M. M. Struys, et al. Comparison of Plasma Compartment versus two Methods for Effect Compartment-Target Controlled Infusion for Propofol. Anesthesiology. 2000, 92, pp. 399-406.
c. J.H. Seo, et al. Influence of a modified propofol equilibration rate constant (Ke0) on the effect-site concentration at loss and recovery of consciousness with the Marsh model. Anaesthesia, 2013.
d. R.F. Simoni, et al. Clinical Evaluation of two Ke0 in the same pharmacokinetic Propofol Model: Study on Loss and Recovery of Consciousness Review of Brasilian Anesthesiology. 61, 2011, 4, pp. 397- 408.
e. Gepts, Shafer, Camu, Stanski, Woestenborghs, Van Peer, Heykants. Linearity of pharmacokinetics and model estimation of sufentanil. Anesthesiology 1995, 83, pp. 1194-1204.
f. J.C. Scott, K.V. Ponganis, D.R. Stanski. EEG Quantification of narcotic effect: the comparative pharmacodynamics of fentanyl and alfentanil. Anesthesiology, 1985, 62, pp. 234-241.
g. J.C. Scott, D.R. Stanski. Decrease Fentanyl and Alfentanil dose requirements with age. A simultaneous pharmacokinetic and pharmacodynamic evaluation. The Journal of Pharmacology And Expermimental Therapeutics, 1987, N 53855/1 vol.240 n°1.
h. A. Absalom, G. Kenny. ‘Paedfusor’ pharmacokinetic data set. British Journal of Anesthesia, 2005, 95, 1, p. 110.
i. B.K. Kataria et al. The pharmacokinetics of propofol in children using three different data analysis approaches. Anesthesiology, 1996, 80, 1, pp. 104- 122.
* if patient age < 13 years
** if patient age is between 13 and 16 years
*** if patient age = 13 years
**** if patient age = 14 years
***** if patient age = 15 years
****** if patient age = 16 years
NOTE Be especially careful when selecting a TCI model as this selection leads to different flow rate patterns. Only use TCI if you understand its use.
NOTE Be cautious when selecting an appropriate TCI model to ensure patient safety and optimal dosing as different TCI models have different behaviour.
Eleveld TCI:
Model development
The Eleveld TCI models are pharmacokinetic-pharmacodynamic (PK-PD) models designed for propofol and remifentanil. They were developed to provide a broad application across different patient populations, from neonates to adults. Unlike previous models, such as Schnider or Marsh, Eleveld does not rely on Lean Body Mass (LBM) calculations, which means it can accommodate a wider range of patient weights without artificial constraints as allometric scaling is used.
Propofol model (1)
The 2018 Eleveld propofol TCI model was created using bispectral index (BIS) as the targeted endpoint, ensuring precise control over the depth of anaesthesia. It incorporates adjustments for the enhanced effect of delivering propofol alongside an opioid, making it suitable for both anaesthesia (propofol + opioid) and sedation (propofol only).
Study methods:
Propofol PK data was obtained from 30 previously published studies, five of which also contained BIS observations. A PK-PD model was developed using NONMEM. Weight, age, post-menstrual age (PMA), height, sex, BMI, and presence/absence of concomitant anaesthetic drugs were explored as covariates. The predictive performance was measured across young children, children, adults, elderly, and high-BMI individuals, and in simulated TCI applications.
Study results:
Overall, 15,433 propofol concentration and 28,639 BIS observations from 1,033 individuals (672 males and 361 females) were analysed. The age range was from 27 weeks PMA to 88 yr, and the weight range was 0.68-160 kg. The final model uses age, PMA, weight, height, sex, and presence/absence of concomitant anaesthetic drugs as covariates. A 35-yr-old, 170 cm, 70 kg male (without concomitant anaesthetic drugs) has a V1, V2, V3, CL, Q2, Q3, and ke0 of 6.28, 25.5, 273 litres, 1.79, 1.75, 1.11 litres min-1, and 0.146 min-1, respectively. The propofol TCI administration using the model matches well with recommendations for all age groups considered for both anaesthesia and sedation.
Remifentanil model (2)
The 2017 Eleveld Remifentanil TCI model is an allometric pharmacokinetic-pharmacodynamic (PK-PD) model designed to predict remifentanil concentration and effect across a wide range of patient ages and weights.
Key Features of the Model:
-
Uses age, weight, and sex as covariates to improve accuracy.
-
Provides better predictive performance than the Minto model, especially across diverse patient populations.
-
Effect compartment rate constant (ke0) decreases with age, meaning older patients experience slower drug onset.
Study methods:
Remifentanil pharmacokinetic data were obtained from three previously published studies of adults and children, one of which also contained pharmacodynamic data from adults. NONMEM was used to estimate allometrically scaled compartmental pharmacokinetic and pharmacodynamic models. Weight, age, height, sex, and body mass index were explored as covariates. Predictive performance was measured across young children, children, young adults, middle-aged, and elderly.
Study results:
Overall, 2,634 remifentanil arterial concentration and 3,989 spectral-edge frequency observations from 131 individuals (55 male, 76 female) were analysed. Age range was 5 days to 85 yr, weight range was 2.5 to 106 kg, and height range was 49 to 193 cm. The final pharmacokinetic model uses age, weight, and sex as covariates. Parameter estimates for a 35-yr-old, 70-kg male (reference individual) are: V1, 5.81 l; V2, 8.82 l; V3, 5.03 l; CL, 2.58 l/min; Q2, 1.72 l/min; and Q3, 0.124 l/min. Parameters mostly increased with fat-free mass and decreased with age. The pharmacodynamic model effect compartment rate constant (ke0) was 1.09 per minute (reference individual), which decreased with age.
Eleveld study design
The Eleveld TCI model studies as compared to other common TCI model studies are summarised below (click on table to expand).

Eleveld drug options:
Propofol and remifentanil
Agilia SP TIVA syringe pumps with firmware version 4.3 and above also have the option of Eleveld TCI pharmacokinetic models. Any Agilia SP TIVA syringe pump with a firmware version below 4.3 can be upgraded to allow Eleveld TCI on your existing syringe pump.
NOTE Eleveld TCI is not included as standard in your purchase. To have Eleveld TCI enabled or configured for your device, whether this be a new device order or an upgrade of an existing device, requires purchase of the “ELEVELDUPG” product code. Please contact your local Fresenius Kabi Account Manager to request a quote.
The Eleveld TCI model comes in two versions for propofol administration:
-
one for concurrent use with an opioid
-
one without opioid
The version with opioid tends to deliver lower cumulative doses and decreases dose based on the increasing age of the patient.

Why the two options?
With opioids (1):
When propofol is used alongside an opioid, the required dose is lower because the opioid enhances the sedative effect, reducing the amount of propofol needed to achieve the same level of unconsciousness.
Without opioids (1):
If propofol is used alone, a higher dose is required to maintain the desired anaesthetic depth, since there's no opioid to amplify the effect.
Clinical Relevance (1):
The “with opioids” setting is commonly used for general anaesthesia, where both propofol and remifentanil work together to provide sedation and pain control.
The “without opioids” setting is often used for procedural sedation, where only propofol is given, and opioid-induced respiratory depression needs to be avoided.
This dual-option approach ensures optimal dosing for various clinical scenarios, minimising risks and improving patient safety.
Eleveld TCI for remifentanil only comes in one version which does not have a choice of with or without opioid as remifentanil itself is an opioid.
Schnider TCI vs Eleveld TCI:
Propofol
The Eleveld pharmacokinetic model delivers a larger initial bolus than the Schnider one as can be seen below in the comparative cumulative dose graphs.
NOTE Users are advised to adjust the initial target dose accordingly to ensure patient safety and avoid potential overdose.

Tivatrainer (tivatrainer.com), is a drug simulation application designed for medical professionals to help understand intravenous anaesthesia techniques. It provides pharmacokinetic models for various drugs, allowing users to simulate different dosing strategies and their effects.
Using Tivatrainer, a comparison between Eleveld Propofol + opioid, Schnider and Marsh TCI is shown below to give you an idea of how using the various TCI models will be delivered to your patients.
83 kg patient, age 70 years, height 170cm, male, CeT 2.8 mcg/mL at 42 secs

83 kg patient, age 70 years, height 170cm, male, CeT 2.8 mcg/mL at 3 mins 3 secs

83 kg patient, age 70 years, height 170cm, male, CeT 2.8 mcg/mL at 10 mins

Eleveld TCI and age:
Propofol (1)
The Eleveld pharmacokinetic (PK) and pharmacodynamic (PD) TCI model adjusts delivery with respect to age-related changes in drug metabolism and sensitivity.
Slower Metabolism in Older Patients:
As age increases, the model accounts for reduced clearance of propofol, meaning lower infusion rates may be needed to achieve the same effect.
Effect-Site Sensitivity:
Older patients tend to be more sensitive to propofol, so the model adjusts the CeT (effect-site concentration) accordingly.
NOTE Clinical judgement is still essential, especially for fragile patients such as the elderly, as the induction bolus in Eleveld TCI is higher than in other TCI models such as Schnider. If careful dosing is required, titrate in small steps toward the final target.
Using Tivatrainer, a comparison between a 50-year-old, 70-year-old and 90-year-old patient is shown below to give you an idea of how using the Eleveld propofol + opioid TCI model will be delivered to your patients.
75 kg patient, age 50/70/90 years, height 170cm, male, CeT 2.8 mcg/mL at 42 secs
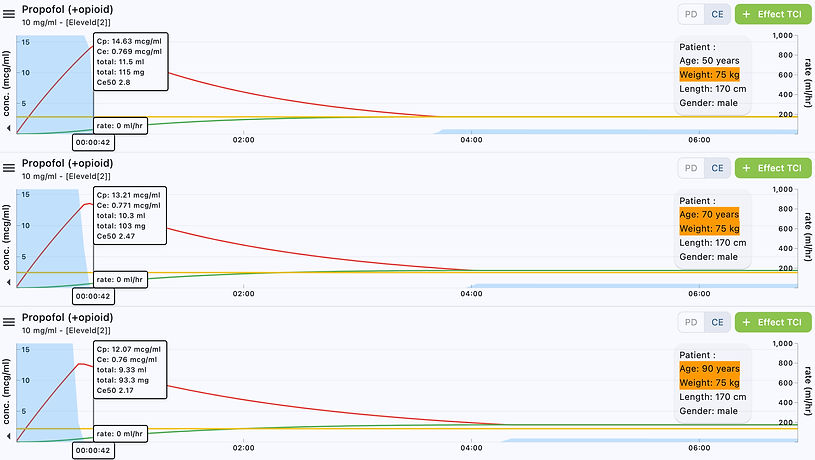
75 kg patient, age 50/70/90 years, height 170cm, male, CeT 2.8 mcg/mL at 5 mins

75 kg patient, age 50/70/90 years, height 170cm, male, CeT 2.8 mcg/mL at 90 mins

Implementation of Eleveld TCI:
Agilia SP TIVA (firmware version 4.3 or above)
Allometric scaling is the study of how biological traits change in proportion to body size and has been used in TCI with many models such as the Marsh TCI pharmacokinetic model for Propofol administration.
The Marsh TCI model was originally developed using paediatric data, but it has been widely used in adults because its pharmacokinetic parameters can be appropriately scaled. The Marsh model applies allometric scaling principles, which adjust for differences in body size and metabolic rates between children and adults.
Allometric scaling is particularly useful in anaesthesia because drug metabolism and clearance do not increase linearly with body weight. Instead, larger individuals often require proportionally lower doses per kilogram compared to smaller patients. This principle helps refine dosing accuracy, especially in obese patients, where traditional models may overestimate or underestimate drug requirements (12).
Unlike some other models, which have upper weight limits due to lean body mass calculations, Eleveld does not impose such restrictions as it incorporates allometric scaling to adjust drug dosing based on body size. This flexibility makes it more adaptable across different patient populations such as obese patients (13).
Increasing weight increases the dose but not in a linear fashion with the Eleveld TCI model. As the Eleveld algorithm does not use Lean Body Mass (LBM), there are no limits in the algorithm on weight (1).
However, while allometric scaling helps adjust drug dosing based on body size, clinical judgement is still essential, especially for fragile patients or patients who were outside the initial patient cohort upon which the algorithm was developed.
A summary of how age, weight and height ranges have been implemented on common TCI syringe pumps is shown below for various pharmacokinetic TCI models.

The Eleveld TCI algorithm does not impose restrictions on age, height or weight as it incorporates allometric scaling to adjust drug dosing based on body size.
For this reason, Fresenius Kabi has chosen to allow a wider range of limits on the Agilia SP TIVA syringe pump than was clinically evaluated in the initial 2017 publication for Eleveld remifentanil, initial 2018 publication for Eleveld propofol and subsequent prospective clinical validation of the propofol model published in 2021.
This allows for a wide range of age, height and weight limits for Eleveld TCI propofol and remifentanil administration as shown below which can be tailored to meet your clinical needs.

* Covariates shown are for Propofol and Remifentanil Eleveld TCI as implemented on the Agilia SP TIVA using firmware version 4.3.
NOTE All clinicians are responsible for ensuring that when a Target-Controlled Infusion (TCI) pharmacokinetic model is applied to a patient outside of clinically validated ranges for age, height, or weight, appropriate titration to target in small steps is used to optimise safety and efficacy.
Using Tivatrainer, various patient examples outside of the clinically validated range for weight are shown below for Propofol + opioid administration to give you an idea of how using the Eleveld TCI model will be delivered to your patients.
150/200/250 kg patient, age 50 years, height 170cm, male, CeT 2.8 mcg/mL at 2 mins

150/200/250 kg patient, age 50 years, height 170cm, male, CeT 2.8 mcg/mL at 3 mins 15 secs

150/200/250 kg patient, age 50 years, height 170cm, male, CeT 2.8 mcg/mL at 4 mins 30 secs

150/200/250 kg patient, age 50 years, height 170cm, male, CeT 2.8 mcg/mL at 10 mins

150/200/250 kg patient, age 50 years, height 170cm, male, CeT 2.8 mcg/mL at 60 mins

Eleveld TCI CeT setting:
Propofol
For the final PK-PD model, the relationship between effect site concentration and predicted BIS, and the relationship between age and effect-site concentration for 50% (anaesthesia) and 10% (sedation) drug effect (Ce50 and Ce10) are shown below (1).
NOTE Be aware of the authors comments below on how these targets were calculated and ensure if using these targets that they are clinically appropriate for each of your patients.
“For anaesthetic concentration targets, we assumed the presence of concomitant anaesthetic techniques (e.g. opioids or local anaesthesia), whereas for sedation targets we assumed their absence. For anaesthesia targets of the Ce50, the expected BIS value would be 93*(1-0.5)=47, whilst for MAC sedation targets of the Ce10 it would be 93*(1-0.1)=84. Because Ce50 and Ce10 vary with age, the specific target concentration depends on age.” (1)

Anaesthesia targets in obese adults
For simulation of anaesthesia TCI for obese adults, the study (1) assumed an age of 35 yr, height of 170 cm, and varied weight to achieve BMI values of 30-50. The target effect-site concentration was the same as for non-obese individuals, the age-adjusted Ce50.
For the Diprifusor PK and Schnider and colleagues (19) PK-PD models, the target concentration was 4 mg ml-1.
The FDA drug product label for propofol does not specifically address drug dosing in obesity, so in the study they adjusted adult (non-obese) recommendations by the equation of Servin and colleagues (20), in which doses for obese individuals are based on ideal body weight (IBW) plus 40% of excess weight, where IBW = 22 * HGT².
Thus, the weight used for dosing was:
dose weight (kg) = IBW + 0.4(WGT - IBW)
This approach serves to decrease dose per kilogram body weight in obese individuals as body weight exceeds IBW. Propofol administration from TCI simulations was compared with the adjusted dose recommendations.
Simple and intuitive workflow
The user interface of the Agilia SP TIVA has been designed, and tested in the hands of anaesthetists, to enable simple setup and quick initiation.
Below is an example workflow from power on to Eleveld TCI delivery.

Review the clinical papers
Prospective clinical validation of the Eleveld propofol pharmacokinetic-pharmacodynamic model in general anaesthesia.
Citation: Vellinga R, Hannivoort LN, Introna M, Touw DJ, Absalom AR, Eleveld DJ, Struys MMRF. Prospective clinical validation of the Eleveld propofol pharmacokinetic-pharmacodynamic model in general anaesthesia. Br J Anaesth. 2021 Feb;126(2):386-394. doi: 10.1016/j.bja.2020.10.027. Epub 2020 Dec 13. PMID: 33317804.
URL: https://pubmed.ncbi.nlm.nih.gov/33317804/
Pharmacokinetic-pharmacodynamic model for propofol for broad application in anaesthesia and sedation.
Citation: Eleveld DJ, Colin P, Absalom AR, Struys MMRF. Pharmacokinetic-pharmacodynamic model for propofol for broad application in anaesthesia and sedation. Br J Anaesth. 2018 May;120(5):942-959. doi: 10.1016/j.bja.2018.01.018. Epub 2018 Mar 12. Erratum in: Br J Anaesth. 2018 Aug;121(2):519. doi: 10.1016/j.bja.2018.05.045. PMID: 29661412.
URL: https://pubmed.ncbi.nlm.nih.gov/29661412/
An Allometric Model of Remifentanil Pharmacokinetics and Pharmacodynamics.
Citation: Eleveld DJ, Proost JH, Vereecke H, Absalom AR, Olofsen E, Vuyk J, Struys MMRF. An Allometric Model of Remifentanil Pharmacokinetics and Pharmacodynamics. Anesthesiology. 2017 Jun;126(6):1005-1018. doi: 10.1097/ALN.0000000000001634. PMID: 28509794.
Have any questions?
Please contact us using the form below for more details on Agilia SP TIVA, Eleveld TCI or to arrange an evaluation or upgrade to your existing Agilia SP TIVA pumps.
Note, this service is only available to residents of Australia and New Zealand.
References
(1) Eleveld DJ, Colin P, Absalom AR, Struys MMRF. Pharmacokinetic-pharmacodynamic model for propofol for broad application in anaesthesia and sedation. Br J Anaesth. 2018 May;120(5):942-959. doi: 10.1016/j.bja.2018.01.018. Epub 2018 Mar 12. Erratum in: Br J Anaesth. 2018 Aug;121(2):519. doi: 10.1016/j.bja.2018.05.045. PMID: 29661412.
(2) Eleveld D, Proost J, Vereecke HEM, et al. An allometric model of remifentanil pharmacokinetics and pharmacodynamics. Anesthesiology 2017; 126:1005–1018.
(3) Vellinga R, Hannivoort LN, Introna M, Touw DJ, Absalom AR, Eleveld DJ, Struys MMRF. Prospective clinical validation of the Eleveld propofol pharmacokinetic-pharmacodynamic model in general anaesthesia. Br J Anaesth. 2021 Feb;126(2):386-394. doi: 10.1016/j.bja.2020.10.027. Epub 2020 Dec 13. PMID: 33317804.
(4) Kataria BK, Ved SA, Nicodemus HF, Hoy GR, Lea D, Dubois MY, Mandema JW, Shafer SL. The pharmacokinetics of propofol in children using three different data analysis approaches. Anesthesiology. 1994 Jan;80(1):104-22. doi: 10.1097/00000542-199401000-00018. PMID: 8291699.
(5) Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991 Jul;67(1):41-8. doi: 10.1093/bja/67.1.41. PMID: 1859758.
(6) Schnider TW, Minto CF, Shafer SL, Gambus PL, Andresen C, Goodale DB, Youngs EJ. The influence of age on propofol pharmacodynamics. Anesthesiology. 1999 Jun;90(6):1502-16. doi: 10.1097/00000542-199906000-00003. PMID: 10360845.
(7) Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991 Jul;67(1):41-8. doi: 10.1093/bja/67.1.41. PMID: 1859758.
(8) Struys MM De Smet T Depoorter Bet al., Comparison of plasma compartment versus two methods for effect compartment–controlled target-controlled infusion for propofol, Anesthesiology, 2000, vol. 92 (pg. 399-406).
(9) Cortínez LI, Anderson BJ, Penna A, Olivares L, Muñoz HR, Holford NH, Struys MM, Sepulveda P. Influence of obesity on propofol pharmacokinetics: derivation of a pharmacokinetic model. Br J Anaesth. 2010 Oct;105(4):448-56. doi: 10.1093/bja/aeq195. Epub 2010 Aug 14. PMID: 20710020.
(10) Cortínez LI, De la Fuente N, Eleveld DJ, Oliveros A, Crovari F, Sepulveda P, Ibacache M, Solari S. Performance of propofol target-controlled infusion models in the obese: pharmacokinetic and pharmacodynamic analysis. Anesth Analg. 2014 Aug;119(2):302-310. doi: 10.1213/ANE.0000000000000317. PMID: 24977639.
(11) Minto, C.F., et al. "Influence of Age and Gender on the Pharmacokinetics and Pharmacodynamics of Remifentanil. I. Model development." Anesthesiology, 1997: Vol. 86, pp. 10-23.
(12) Eleveld, Douglas J. Ph.D.; Koomen, Jeroen V. Ph.D.; Absalom, Anthony R. M.B.Ch.B., F.R.C.A., M.D.; Su, Hong M.Sc.; Hannivoort, Laura N. M.D., Ph.D.; Struys, Michel M. R. F. M.D., Ph.D., F.R.C.A.. Allometric Scaling in Pharmacokinetic Studies in Anesthesiology. Anesthesiology 136(4):p 609-617, April 2022. | DOI: 10.1097/ALN.0000000000004115.
(14) Agilia SP TIVA (WiFi) Syringe Infusion Pumps. Applicable to software version 4.3. Instructions For Use. 19176-0_ifu_agilia_sp_tiva_eng.
(15) BD Alaris neXus syringe pump. Directions For Use. Ref: PKneXus1.BDDF00329 Issue 2.
(16) Space Plus Perfusor. Instructions for use en Version 1.0 English. Valid for software 019D. 39012107_Perfusor_spaceplus_en_IFU_0724 v31(SwD).
(17) Fresenius Kabi data on file.
(18) Engbers, F.H.M. and Dahan, A. (2018), Anomalies in target-controlled infusion: an analysis after 20 years of clinical use. Anaesthesia, 73: 619-630. https://doi.org/10.1111/anae.14212.
(19) Schnider TW, Minto CF, Gambus PL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 1998; 88: 1170-82.
(20) Servin F, Farinotti R, Haberer JP, Desmonts JM. Propofol infusion for maintenance of anesthesia in morbidly obese patients receiving nitrous oxide. A clinical and pharmacokinetic study. Anesthesiology 1993; 78: 657-65.
